SOLUBILITY
Home » SOLUBILITY
Subscribe to our Newsletter
SOLUBILITY
SOLUTION
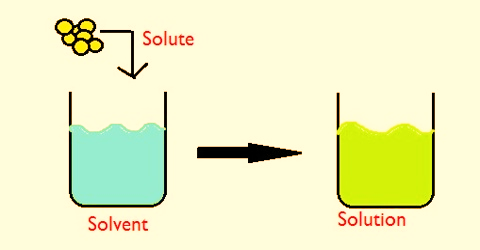
A solution is a mixture of two or more substances in a single phase. At least two substances must be mixed in order to have a solution.
The substance in the smallest amount and the one that dissolves or disperses is called the SOLUTE.
The substance in the larger amount is called the SOLVENT. In most common instances water is the solvent.
The gases, liquids, or solids dissolved in water are the solutes.
SOLUTE + SOLVENT = SOLUTION
Salt + water = Salt solution
· We know that water is the solvent of a large number of substances. Sugar, salt, alum, blue vitriol, potassium-permanganate, etc. are soluble in water, while wax, sand, paints, etc. are insoluble in it.
· There are substances which do not dissolve in water, but dissolve in other liquids. Candle-wax, grease, paint, etc. do not
dissolve in water but dissolve in kerosene oil or petrol.
· A liquid dissolves not only solids but other liquids and gases also. Water dissolves air and carbon-di-oxide. Fish and other water animals take in this dissolved air.
· When a soda or cold drink is opened, we see carbon-di-oxide fizzling out. Liquid paint dissolves in kerosene oil and alcohol in water.
FACTORS THAT EFFECT THE SOLUBILITY
1. EFFECT OF TEMPERATURE
Usually when we heat up a solution, the amount of solubility of the solute in the solvent increases.
This happens because when we heat the solution, the molecules of the solvent get the heat energy and so they start moving faster and hence away from each other.
This causes more space between the molecules of the solvent, and so more solute molecules can take up the empty space between the solvent molecules.
If your friend was mixing sugar and water, she would be able to dissolve a lot more sugar into hot water rather than cold water.
2. EFFECT OF SIZE
If the size of the solute is smaller then it dissolves faster. For eg. If we have crushed sugar then it gets dissolved faster than the sugar cubes.
3. EFFECT OF QUANTITY
When the quantity is more then it takes more time for the solute to get dissolved in the solution. For eg. If we have more sugar, then it takes more time to dissolve than the lesser amount.
Saturation:
A solute that normally dissolves in water, like sugar or salt, won’t continue to dissolve once it reaches saturation point. This is when the maximum amount of the solute has been dissolved into the water.
BOOKS
We have our e-books published on Amazon for Grade 3 and Grade 4. The books serve as an important guide for Science Olympiads organized by SOF, Silverzone, Unified Council and others. Books are designed to help students understand key science concepts.
The key highlights of the book are:
· Well explained topics
· Use of diagrams and images for
students to visualize
· Test exercise after each chapter for self-assessment and evaluation
· Interesting facts sections spread across the book
Here are the links:





