HEAT CONDUCTIVITY
Home » HEAT CONDUCTIVITY
Subscribe to our Newsletter
CONDUCTIVITY OF HEAT
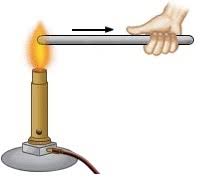
If we take two rods, one of iron and another of wood and place one end of each in fire, on touching their other ends, the iron rods feels hot while the wooden one does not. The first end of the wooden rod actually catches fire. That means the iron rod passes heat from one end to another while the wooden rod does not.
The ability of a substance to allow the flow of heat through its body is called conductivity of heat or thermal conductivity.
The substances which allow the heat to pass through them easily are called good conductors of heat.
The substance which do not allow the heat to pass through them easily are called bad conductors of heat.
Wood, plastic, etc. are bad conductors of heat while metals like copper, silver, gold, iron, aluminium, etc. are good conductors.
EXPANSION AND CONTRACTION
Getting Hotter = Getting Bigger: Thermal Expansion
We’ll start with gases. The idea behind thermal expansion is that gases expand as the temperature increases.
The opposite of expansion is contraction. If things expand with the addition of heat, it makes sense that they contract when heat is removed.
If you remove enough heat from a gas it will become a liquid. Liquids can turn into solids with further cooling.
BIMETALLIC STRIP
A bimetallic strip consists of two different materials with different expansion coefficients that are bonded together.
For example, for brass and steel
When the strips are at room temperature: There is no expansion or contraction
When the bimetallic strip is heated: Since brass expands more than steel, so it has greater length and hence it comes on the outer side of the curve.
When this bimetallic strip is cooled: Since brass contracts more than the steel, so brass strip becomes shorter in length, and hence it curves with the steel on the outside.
BOOKS
We have our e-books published on Amazon for Grade 3 and Grade 4. The books serve as an important guide for Science Olympiads organized by SOF, Silverzone, Unified Council and others. Books are designed to help students understand key science concepts.
The key highlights of the book are:
· Well explained topics
· Use of diagrams and images for
students to visualize
· Test exercise after each chapter for self-assessment and evaluation
· Interesting facts sections spread across the book
Here are the links:





